January 2021 CKD Insider - Start the New Year Right!
- Jan 30, 2021
- 7 min read
Welcome to the Chronic Kidney Disease Newsletter. If you are person with chronic kidney disease (CKD) on dialysis or helping care for someone who is, this newsletter was created for you! The content is meant to keep you and/or a family member up to date on the latest information to help you manage your health now or in the near future in consultation with your physician.
In this month’s CKD Insider: COVID-19 vaccination planning & latest antibody treatments, Research in the News, Clinical Study participation (AV graft), new Genomic registry, recently approved medication, and Fun Tip of the Day.
COVID-19 Questions & Answers
How is prioritization determined for COVID-19 vaccination? How does it work in my state?
Each state government in the United States of America determines its own prioritization strategy based on the needs of the local population. With that said, the CDC Advisory Committee on Immunization Practices (ACIP) recommendations issued in December 2020 has been a guide for the states to use. The ACIP called for targeting limited vaccine doses to health care workers and long-term care facility residents in phase 1a and people over age 75 and frontline essential workers in phase 1b. While many states have followed the ACIP guidelines, some have not.
You should first check with your state and local governments to determine where you are on the priority list, many states include those with chronic conditions such as those with chronic kidney disease in their priorities list. If your governor or local officials send out an email or provide updates via phone texts sign up for these so you are aware of any changes as they happen.
The nonprofit Kaiser Family Foundation has also been tracking how each state is prioritizing. If you are not sure on the priority order for your state look at the KFF table here. As of today a person who is vaccinated could still potentially spread the virus, so if you have been vaccinated it is important to continue acting as if you are contagious and take the appropriate precautions.
What are the latest treatments available if I am exposed to COVID-19?
Eli Lilly’s neutralizing monoclonal antibody bamlanivimab (LY-CoV555) was authorized for emergency use (EUA) by the U.S. Food and Drug Administration (FDA) for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40kg) who are at high risk for progressing to severe COVID-19 and/or hospitalization this includes those with certain chronic medical conditions and those over 65 years of age.
The EUA was granted in November, this month Eli Lilly released new results from their clinical trial on wheels study where they retrofitted recreational vehicles to create a traveling clinical trial site which allowed them to travel to nursing homes when there was an outbreak and incorporate the people there into their clinical trials for this therapy. Eli Lilly announced that their recent data demonstrates that this antibody treatment can prevent COVID-19 infection symptoms. Read more details here.
This treatment is given intravenously which means dialysis centers are in a good position to provide this treatment as long as they have created a separate COVID-19 area in the facility and have the therapy on hand. Ask your dialysis center if they are set up for this.
Patients and physicians can visit covid.infusioncenter.org or the HHS Therapeutic Distribution locator to find a potential treatment location, or visit combatcovid.hhs.gov to find out more about antibody therapy.
For further details please read the FDA Letter of Authorization, Fact Sheet for Healthcare Providers, and Fact Sheet for Patients, Parents, and Caregivers (English) (Spanish).
What is a neutralizing antibody?
Neutralizing antibodies defend cells from pathogens like viruses that cause disease. They are produced naturally as part of the immune response, and can also be made in the laboratory to mimic the immune system response.
Convalescent plasma treatments
If you have heard of people who recovered from COVID-19 donating their convalescent plasma to help treat someone else with COVID-19, the hope is that there are enough antibodies to the SARS-CoV-2 virus including neutralizing antibodies to make a difference in the outcome for another patient. This is why the FDA approved its use as an investigational product with the recommendation of measuring neutralizing antibodies. Learn more about the recommendations for investigational COVID-19 Convalescent Plasma here and about the process of donating here.
Recovered from COVID-19 and want to donate your plasma?
Find donation location centers here.
Reflecting on the COVID-19 vaccine rollout to the public
While the vaccine rollout has not been as smooth or as fast as we need it to be, we should take a moment to reflect on how far we have come in a very short time frame. New vaccine development and approvals for public use typically can take anywhere from 10-20 years, with the fastest on record being the mumps vaccine in the 1960s which took 4 years to get to the market.
The ability to get the first SARS-CoV-2 vaccines to market within one year, is a feat that was accomplished by the focused efforts of many groups around the globe. Starting with the scientific researchers who had been studying SARS & MERS for years prior to SARS-CoV-2, the scientists who quickly sequenced the SARS-CoV-2 genome and shared it globally and shared new developments on how the virus works as quickly as they could without concern over keeping the information for a premier publication. New advances in how to make vaccines and manufacturing infrastructure capabilities, in combination with the enormous amount of government and private funding support and our regulatory agency’s all hands-on deck focus on providing emergency use authorizations for the vaccines while maintaining the clinical trial rigor and evaluation we expect all contributed to where we are today. A big thank you to all the people involved!
It is also important to note that FDA issued Emergency use authorizations require vaccine companies to conduct follow-up surveys to look for side effects and continuing efficacy.
If you want to learn more details Nature magazine has published many good articles on this topic, and if you want to see what is involved in a typical vaccine development and approval process you can view this on the CDC website here.
Your Fun Tip of the Day!
Start the New Year with a Positive Mindset!
This past year has been a challenging year for most of us. If you haven’t taken time to reflect during this time, there is no time like the present!

1. Take a look back on the last 4-5 years, use your calendar, old emails and pictures you’ve taken to help remind you of the things you have done, who you were with and where you went. If you feel inspired, you can use these images to create a digital memory book or video as a keepsake.
2. Next write down your top activities from that time period including accomplishments and challenges you had, what you didn't like and what you were most proud of. What meant the most to you – was it a business/job related activity, time with family/friends, a creative endeavor, physical challenge that you achieved?
3. Circle what was the most rewarding for you from the list above - what inspired you and what brought you joy.
4. Now make your 2021 goals using your previous circled list as a reference focusing on new activities that you can do this year that bring you the same sense of inspiration, joy and happiness as you had previously. And then, make it happen!
5. Clarity is power – once you are clear on what you want to achieve and feel in the coming year, it will all come together, hopefully with a little help from your family and friends!
Research in the News

What it is: A large population-based cross sectional three generation family study of 155,911 participants in the Netherlands looked at creatine and estimated GFR levels in CKD disease family members. They found that a positive family history was strongly associated with increased risk of CKD, with moderate to high heritability of kidney traits and related biomarkers. These results indicate an important role of genetic factors in CKD risk.
Why it’s important to you: Understanding the risks to your biological family members will help you guide them and remind them to keep track of key blood test results over time and make appointments with a nephrologist as needed.
Clinical Study Participation
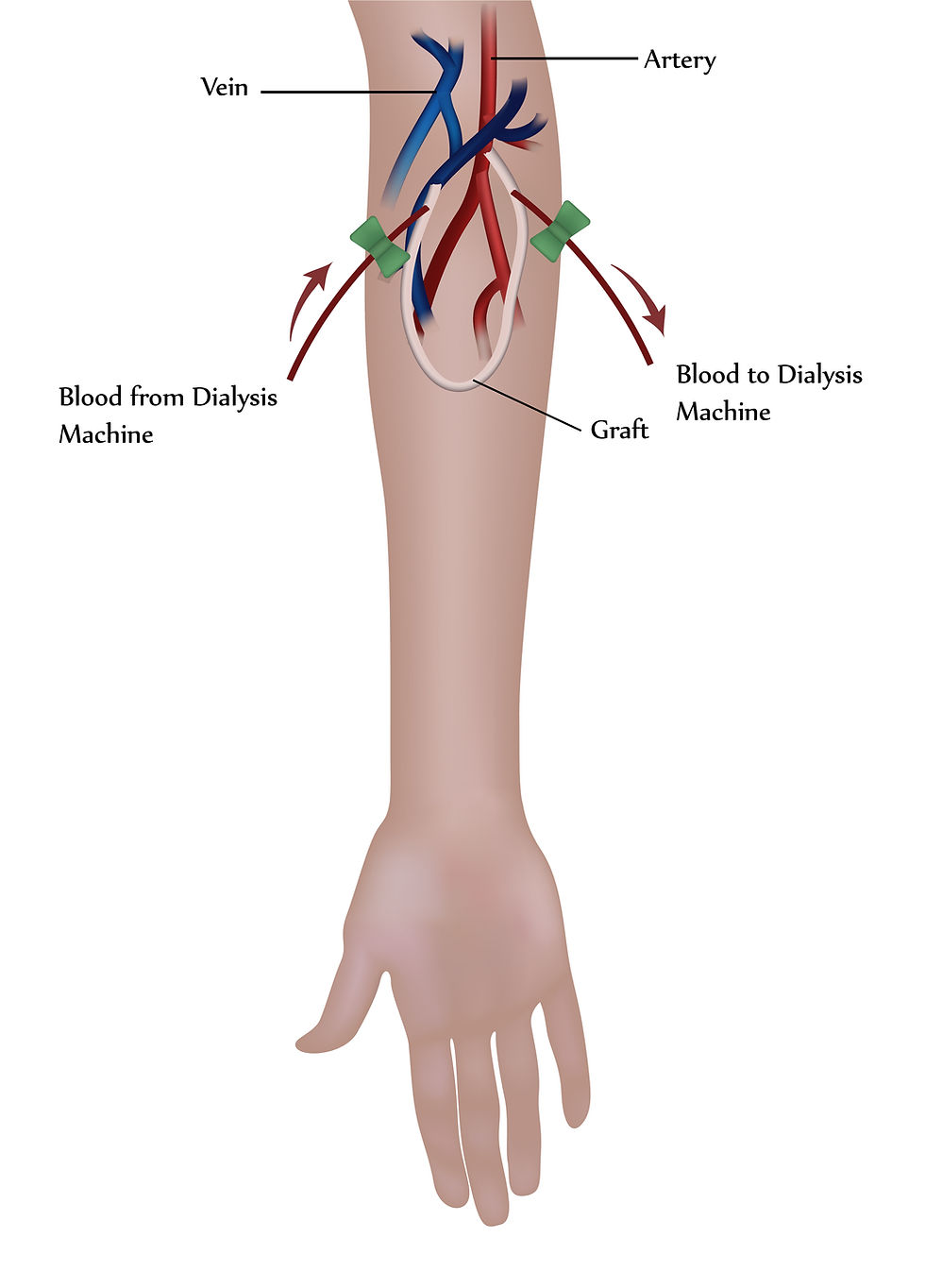
AV refers to the connection between an artery and a vein. Your arteries carry oxygen-rich blood from your heart and lungs to the rest of your body. Your veins then carry oxygen-depleted blood back to the heart and lungs
What it is: An investigational use only AV graft developed by InnAVasc Medical. It is designed to make the graft easier to locate under your skin, safer to stick for dialysis, more durable, with the goal of minimizing risk of complications from bleeding and allowing for immediate use to reduce catheter time.
What it means for you: Both an AV fistula and an AV graft have advantages and disadvantages. If an AV fistula doesn't work for you, an AV graft is often the next best option. This study, if you are eligible gives you an option to not only have an improved AV graft functionality for yourself but can help others as well.
If you or your family member are interested in being part of this clinical study you learn more here to find a research doctor near you. As of this date, research locations are in Kentucky, Mississippi or Texas and if you qualify would be no-cost to you.
Note: It is currently an Investigational Device, Limited by Federal (or United States) Law to Investigational Use.

What it is: Fresenius Medical Care, one of the leading companies that provides kidney dialysis services via outpatient dialysis centers announced that the company's Frenova division has enrolled the first participants in its new initiative to develop the largest renal-focused genomic registry in the world.
Why it's important to you: If you remember in our October CKD Insider Newsletter we discussed the use of genomics to help advance treatments for kidney disease.
This registry is one piece of the larger puzzle, combining clinical and genetic sequencing data from diverse participants to help scientists better understand how genetic variations in kidney patients affect treatment outcomes ultimately allowing for more precise diagnoses for personalized therapies.
Recently Approved Medications

What it is: The FDA recently approved the use of intravenous and subcutaneous Benlysta to treat lupus nephritis, making it the first therapy ever approved for that indication. Lupus nephritis is a severe form of systemic lupus erythematosus that can cause late-stage renal failure and require dialysis or kidney replacement in the worst cases.
Why it’s important to you: If someone you know has been diagnosed with Lupus nephritis this medication can help them stay away from kidney dialysis or kidney replacement. If you are reading this, you are most likely on dialysis or know someone who is, and therefore recognize the importance of sharing available options that lead away from a path to kidney dialysis. The more we know the more we can share this information with others!
If you found this informative, please sign up for our newsletter and pass this on to anyone you know that could benefit from the information!
Copyright © 2021 | Life4ward, LLC | All rights reserved.




Comments